Signs of Optic Neuropathy Nerve Appearance Review of Optometry Glaucoma

Understanding ONH Dynamics in Glaucoma and Across
Clinicians must know what a healthy optic nerve head looks like and how it functions before they can appreciate the dysfunction that happens with illness.
By Catherine Hogan, OD, and Andrew Rixon, OD
Release Date: July xv, 2021
Expiration Date : July xv, 2024
Estimated Fourth dimension to Consummate Activity: ii hours
Jointly provided by Postgraduate Establish for Medicine (PIM) and Review Educational activity Grouping
Educational Objectives: After completing this activity, the participant should exist better able to:
- Discuss the pathophysiology of optic nerve head disorders.
- Explicate how the optic nervus head functions.
- Identify potentially suspicious optic nerve pathology.
- Diagnose various optic nervus head disorders.
Target Audience: This activeness is intended for optometrists engaged in eye intendance of the optic nerve head.
Accreditation Statement: In support of improving patient intendance, this action has been planned and implemented by the Postgraduate Institute for Medicine and Review Teaching Group. Postgraduate Found for Medicine is jointly accredited by the Accreditation Quango for Continuing Medical Education, the Accreditation Quango for Chemist's Education, and the American Nurses Credentialing Center, to provide standing educational activity for the healthcare squad. Postgraduate Institute for Medicine is accredited past COPE to provide continuing educational activity to optometrists.
Reviewed past: Salus University, Elkins Park, PA
Faculty/Editorial Lath:Catherine Hogan, OD, and Andrew Rixon, OD
Credit Statement: This course is COPE canonical for 2 hours of CE credit. Activity #122080 and course ID 73298-GL . Check with your local land licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements:
Authors:Drs. Hogan and Rixon have no financial interests to disclose .
Managers and Editorial Staff: The PIM planners and managers accept nothing to disclose. The Review Education Group planners, managers and editorial staff have nothing to disclose.
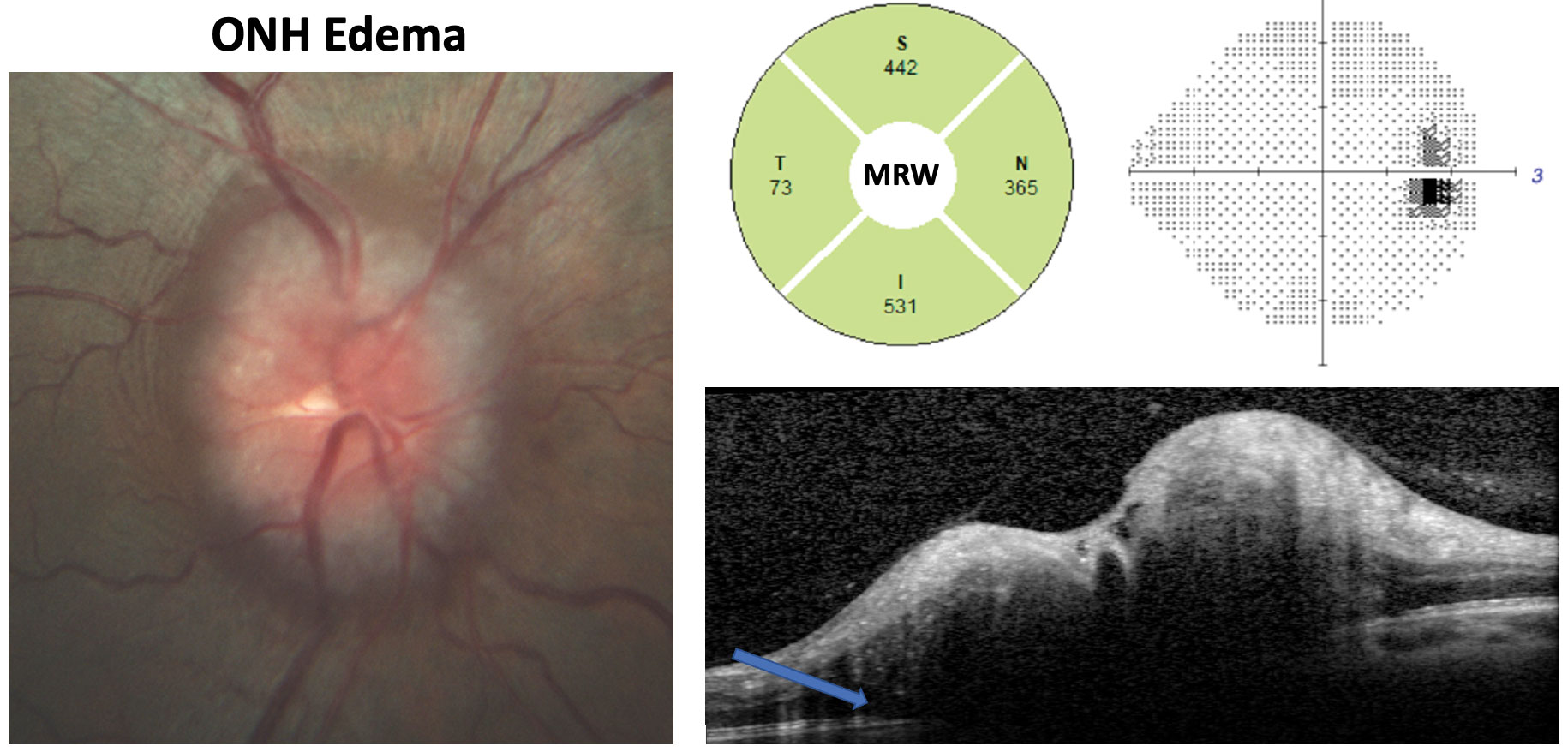 |
| This IIH patient has an opening ICP of 42cm H2O. Fundus photography shows Frisen course three edema. Note the substantially increased MRW, enlarged blind spot on the grayscale plot and the OCT cross-department showing subretinal hyporeflective infinite (bluish pointer) consistent with ONH edema. Click image to enlarge. |
Thoulaucoma is the near common optic neuropathy institute in adulthood, and the near prevalent course—principal open-angle glaucoma (POAG)—is projected to affect 7.32 million individuals in the United States by 2050. 1 This interpretation is staggering and should encourage ODs to not become conceited or make assumptions about glaucoma diagnosis, especially as the condition is a diagnosis of exclusion.
A litany of non-glaucomatous entities tin also cause optic nerve dysfunction, and although this fact confounds our job as clinicians, agreement primal facets of the optic nerve and employing a systematic diagnostic approach volition assist guide united states to the truth, ultimately maximizing the patient experience.
This review will discuss the pathophysiology and clinical assessment of mutual optic nerve caput (ONH) disorders with a applied accent on their differentiating features.
A Systematic Approach
Clinically, a detailed and systematic stereoscopic analysis of the ONH and its surrounding tissue using the slit lamp and various condensing lenses provides framework for the assessment. Although quantitative variables are important, attention to the qualitative aspects and morphology of the ONH should be the primary indicate of accent, as in that location is considerable quantitative overlap between normal and unhealthy ONHs.v
Understanding how to evaluate the glaucomatous disc is key to differentiating the disease. One of the most repeatable systems y'all can follow for guidance is the 'five Rs' developed as function of FORGE (Focusing Ophthalmology on Reframing Glaucoma Evaluation): (1) observing the scleral ring to identify the boundaries of the optic disc and determine its size (larger vertical disc diameters volition naturally have larger cups), (2) assessing the retinal nerve fiber layer (RNFL) for localized or diffuse loss, (3) examining the neuroretinal rim while specifically evaluating the width, shape and color in all sectors with the premise that on boilerplate the disc will follow the ISNT rule and will not have focal tissue loss, (4) checking for retinal or disc hemorrhages, which often straddle the rim just can exist found at the level of the lamina or in the peripapillary retina and (5) evaluating for a region of peripapillary atrophy (PPA) of chorioretinal tissue specially adjacent to areas where the neuroretinal rim is thinner.v,6 An additional glaucoma feature to be on the picket for is deep or laminar cupping created by tissue remodeling and subsequent excavation beneath the scleral canal.7-9
A Closer Look at the ONH
Recognizing what an ONH looks similar and how it functions allows us to better discriminate between normal and aberrant findings, thus unlocking the ability to more specifically diagnose diseases of the ONH. Functionally, the optic nerve transmits stimuli between the neurosensory retina and the lateral geniculate body and cerebral visual pathways. The ONH refers to the ophthalmoscopically visible anterior surface of the nervus.
The ONH consists primarily of iv components: neural fibers, glial cells, extracellular matrix support and vascular tissue.2-four The neural tissue is formed by the retinal ganglion prison cell axons of the RNFL, originating primarily in the macula and converging radially on the ONH in arcuate bundles, forming a convex periphery and the first layer of the ONH, the surface nerve cobweb layer. This neural tissue appears orange-pinkish on fundoscopy every bit a result of the reflectance of low-cal off of the dense capillary network that supplies the disk surface. These fibers and so bend abruptly, passing through the second layer—the prelaminar layer—which is made up of loose, capillary-containing glial tissue. The previously mentioned cobweb bend creates the primal and concave cup, which appears yellowish as a result of the supporting collagenous fibers found in the tertiary layer, the lamina cribrosa region.
The collagenous lamina cribrosa is made upwards of fenestrated connective tissue lamellae that contain capillaries and are covered in astrocytes. The nervus fiber layer passes through the scleral lamina cribrosa and the fourth layer, the retrolaminar region, prior to exiting the world.two,3 The blood supply to the optic nerve is mainly from the posterior ciliary artery, with the exception of the surface of the nerve which receives its supply from retinal circulation. Importantly, ONH blood supply is sectoral in nature.iv Knowing how the neural and vascular tissue is distributed is disquisitional in recognizing how characteristic patterns of tissue damage tin occur in various ONH disorders.
Employing a systematic method can help minimize diagnostic errors. Studies have shown that general ophthalmologists are twice as probable to underestimate glaucoma compared with glaucoma subspecialists. ONHs most probable to be underestimated were horizontally ovoid in shape and those with moderate PPA. Factors associated with underestimation were failure to correctly course vertical cup-to-disc (C/D) ratios, appraise disc hemorrhages and capture RNFL and rim loss.
ONHs most probable to be identified every bit glaucomatous when they were non were large and had nerves with horizontal or vertical disc tilt, amid other factors.10 Researchers plant that in a grouping of optometric observers, average-sized nerves were correctly identified 90% of the time. However, large ONHs tended to be misidentified as glaucomatous, and even more problematically, small ONHs associated with glaucoma were misidentified as normal, reinforcing the size bias during ONH evaluation that we all need to remain enlightened of.xi
These findings may also be influenced by the lack of structured funduscopic evaluation. A written report monitoring the gaze behavior of trainees and experts when assessing ONHs constitute that trainees, although they spent more than time evaluating ONHs, exhibited no specific gaze pattern regardless of whether the ONHs had suspicious findings or not and mainly concentrated on the optic loving cup. Experts, however, took less time and were more systematic and consistent in their approach, concentrating on areas of interest (rim tissue, RNFL and superior and junior poles) and suspicious structural findings as they presented.12
How does optic nerve damage occur in glaucoma? At present, we know that a number of factors influence the retinal ganglion cells and ONH. These include intraocular pressure (IOP), cerebrospinal fluid pressure (CSFP) and ocular perfusion force per unit area (OPP).13 The complex interrelations amongst these factors consequence in glaucomatous optic neuropathy. Although there is not currently a universal consensus of the exact relationship between these factors, there is a characteristic pattern of glaucomatous ONH impairment that is known, helping to more clearly delineate glaucoma from other ONH disorders.
In essence, glaucoma is a laminopathy, whereas other optic neuropathies are non. The aforementioned term "cupping" can create confusion as it tin be conflated with C/D ratio. In fact, cupping has been proposed to involve two components: prelaminar and laminar.viii Prelaminar cupping affects prelaminar neural tissue, and progressive loss of that tissue will increase the depth and the width of the cup, notably increasing the C/D ratio.
The following is where the distinction betwixt non-glaucoma and glaucomatous cupping occurs. Multiple in vivo and cadaveric studies have shown that what separates glaucomatous ONH damage from other neuropathies is the involvement of both connective tissue and neural tissue. Glaucomatous optic nerves undergo progressive posterior bowing/deportation, disorganization and deformation of the lamina cribrosa and peripapillary scleral connective tissue, in improver to the loss of retinal ganglion cell axons.7-9,14 This results in the laminar or deep cupping consistent with glaucoma.
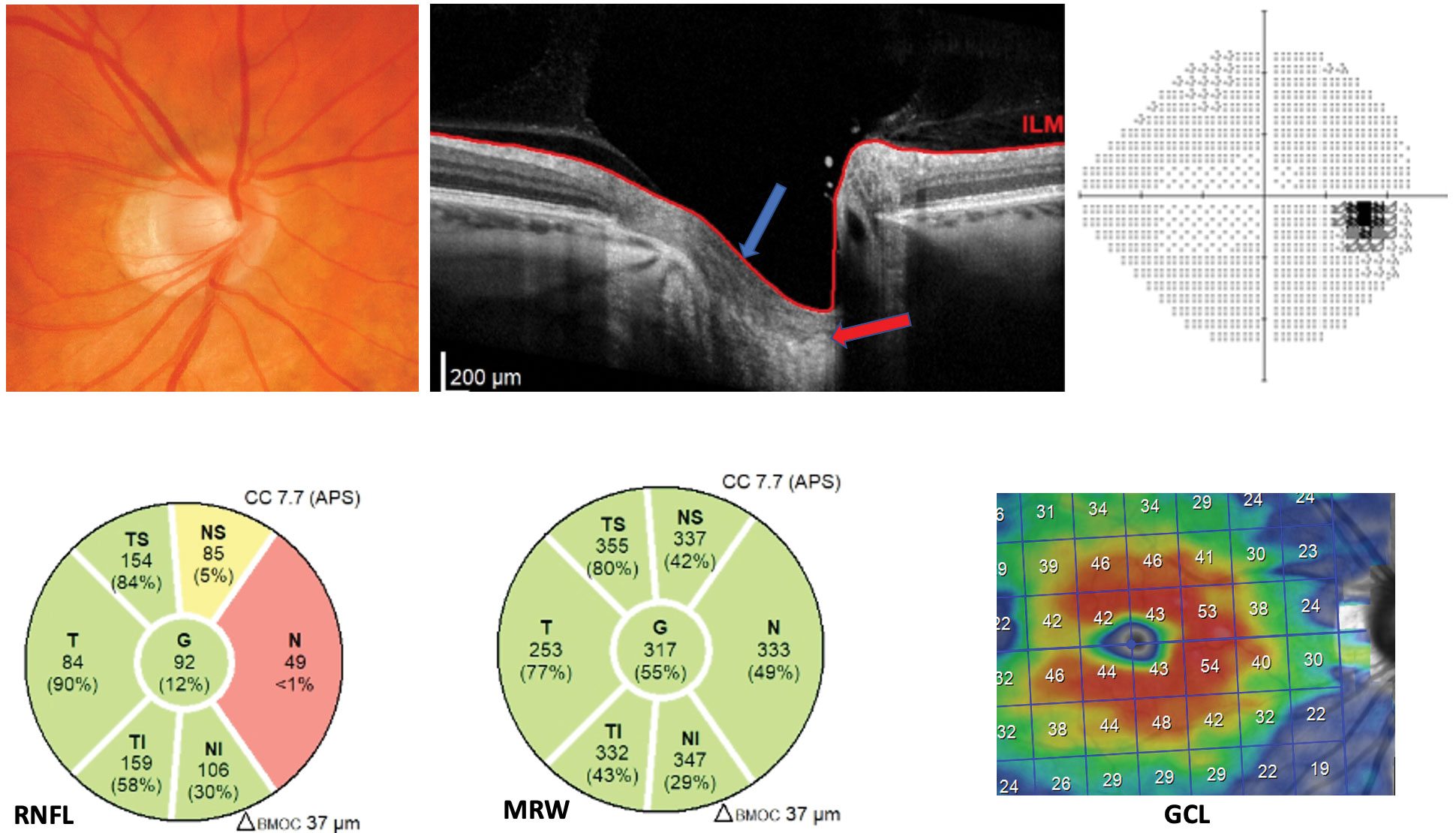 |
| This -7.00D myope was previously classified as a glaucoma suspect. Note the malinsertion and intact pre-laminar tissue (blue pointer) institute on the ONH cantankerous-section. The lamina is marked by the carmine arrow. Additionally, the MRW is unremarkable and the nasal RNFL tissue is thin secondary to temporalized vasculature. Both the ganglion jail cell layer and the visual field are unremarkable. Click image to overstate. |
Clinical Differentiation
Recognizing the morphologic changes institute in glaucoma provides insight for differentiating the disease from other ONH disorders. These differential diagnoses can range from anatomical optic nerve disorders that clinically confound the observer to various neuropathies that cause optic nerve damage via other pathophysiological pathways. Using a combination of detailed clinical examination and ancillary testing will allow the optometric medico to make an informed and accurate diagnosis, treatment and direction plan for the patient.nine This all begins with differentiating glaucoma from its masqueraders, some of which nosotros cover here:
Big optic disc . At first glance, a large optic disc with a correlating large cup commonly leads to glaucoma misdiagnosis. Looking for glaucomatous features instead of zeroing in on cup size lone is an important shift in the process.fifteen Starting time, consider the patient's demographics. Males and African Americans are more likely to have anatomically larger discs.16 Measure the vertical height of the optic disc during funduscopy, and apply the correction factor for magnification per the dioptric power of the lens used. For example, a Volk Super 66D lens or digital 1.0x offers a one.0 correction factor compared with the Volk 90D lens' correction cistron of one.three.17,xviii Big disc height will advise large disc area, a presentation which tin can be misdiagnosed every bit glaucoma.5 Disc area values are populated and confirmed past most Oct modalities.19
Compare quadrants of rim tissue to better recognize how in a big disc, fifty-fifty with a big cup, the rim tissue can be intact. Assess the optic nerve and arcades using the red-free filter to better highlight the RNFL and confirm the lack of wedge defects, a progressive neuropathic disease finding wherein focal RNFL impairment extends from the optic disc and into the papillomacular parcel.twenty
Look within the cup to assess the lamina cribrosa; normal cups not under IOP stressors may have visible laminar dots but should not have laminar deformation/reconfiguration. The clinical cupping associated with glaucoma involves permanent and progressive prelaminar thinning and laminar deformation as a result of IOP stress. This pathophysiologic damage over fourth dimension is absent-minded in anatomically large good for you discs.9
Gauging stability and confirming there is no progressive loss of rim tissue when dealing with anatomically large discs can be accomplished with multiple technologies. RNFL thickness, ganglion cell assay (GCA) and visual field results will remain stable over time in patients with large healthy discs. Nonetheless, RNFL thickness will exist increased in superior, inferior and global quadrants in large discs.21 Spectral-domain (SD) RNFL analysis will also confirm disc area and compare the measured area (in mm2) to a database of normative values. I written report used confocal scanning laser ophthalmoscopy (CSLO) and SD-October to define a large disc area every bit greater than ii.43mm2.xv
Analysis of the rim area as well provides dissimilarity between anatomically big discs and glaucoma. Using CSLO, information technology has been found that progressive glaucomatous optic nerves feel global rim area loss 3.7 times faster than large healthy optic fretfulness.22 OCT analysis of Bruch'southward membrane opening-minimum rim width (BMO-MRW) measures the minimum distance from the BMO to the internal limiting membrane, allowing for more authentic assessment of the neuroretinal rim regardless of disc size.15,23 It is reported that larger disc areas physiologically have larger BMO areas, which contributes to thinner simply stable BMO-MRW results. Therefore, guided progression assay of RNFL and BMO-MRW is paramount to allocate stability vs. progression over fourth dimension.15,24
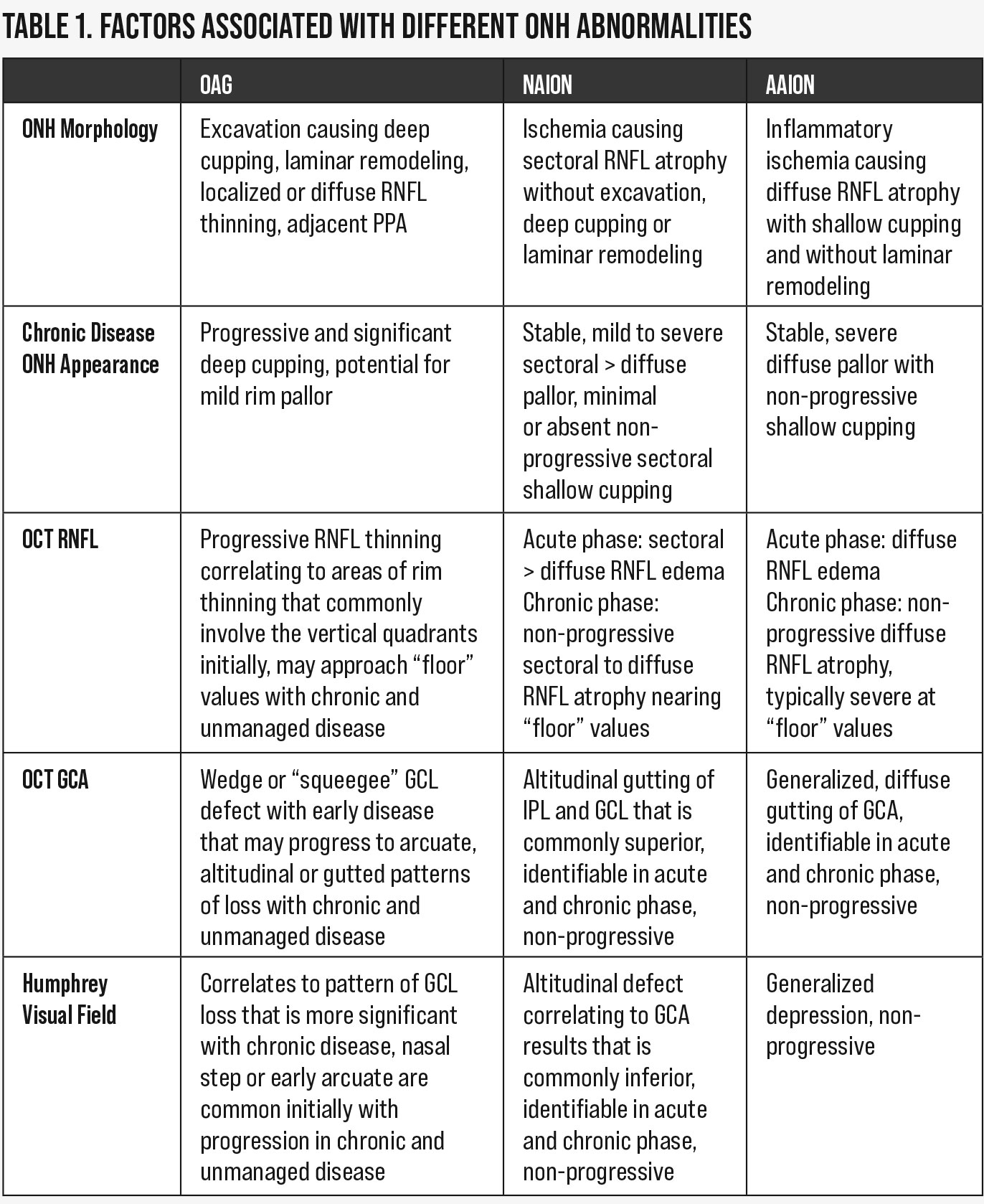 |
| Table 1. Factors associated with unlike ONH abnormalities. Click table to enlarge. |
Tilted disc syndrome (TDS) . This built condition is another anomalous optic nerve condition. It arises from malclosure of the embryonic optic fissure during the second month of gestation. This presents clinically with inferonasally rotated optic discs in tandem with juxtapapillary changes and retinal vascular abnormalities, including next inferonasal crescent or PPA, retinal pigment epithelium and choroidal thinning, posterior staphyloma and situs inversus. The optic disc rotation is non-progressive but can impair the clinician'southward ability to visualize the truthful boundaries of the rim tissue and C/D ratio. The anatomical insertion may lead to a sense of sectoral cupping, but physiologically TDS does not exemplify rim thinning or laminar deformation.ix,25,26
OCT sheds light on what can be clinically misreckoning when differentiating TDS from glaucoma. RNFL results associated with TDS depend on optic nerve morphology and torsion degree merely typically show fake RNFL thinning in nasal quadrants, which are stable over time on glaucoma progression assay (GPA).
GCA and BMO-MRW provide better specificity than RNFL for TDS evaluation. GCA has more uniformity, with less misreckoning results due to anatomy, and shows intact, stable ganglion cell construction over time. BMO-MRW adjusts for individual ONH rim tissue orientation and in TDS, reveals intact, stable neuroretinal rim tissue over fourth dimension.27
Visual field defects in TDS are about ordinarily found in the superior temporal quadrant (call back that inferonasal is the most common disc rotation), with reports of up to 33%.28,29 These defects are non-progressive and do non necessarily respect the horizontal top, emphasizing how TDS physiology spares the nerve fiber layer and lamina cribrosa of thinning.26,28,29
Pseudopapilledema and ONH drusen (ONHD) . The term pseudopapilledema encompasses clinical variations of anomalous ONH elevation, blurring or an indistinct margin advent in the confirmed absence of RNFL edema. Congenital anomalies including tilted and malinserted discs, hypoplasia, myelinated nerve fiber layers, Bergmeister'south papillae and ONHD may create the clinical appearance of an indistinct optic nervus, leading the chief and emergent differential diagnosis to be true optic disc edema.30
Pseudopapilledema impairs complete visualization of rim tissue. Series fundus photography, visual field testing, GCA and OCT with BMO-MRW are valuable tools that, when assessed altogether, provide amend visualization of the anomalous optic nervus structure and a baseline conclusion of function.27 If GPA signals progress over time, the clinician will be able to identify glaucomatous patterns of change that may have been otherwise masked by the anomalous clinical appearance.30,31
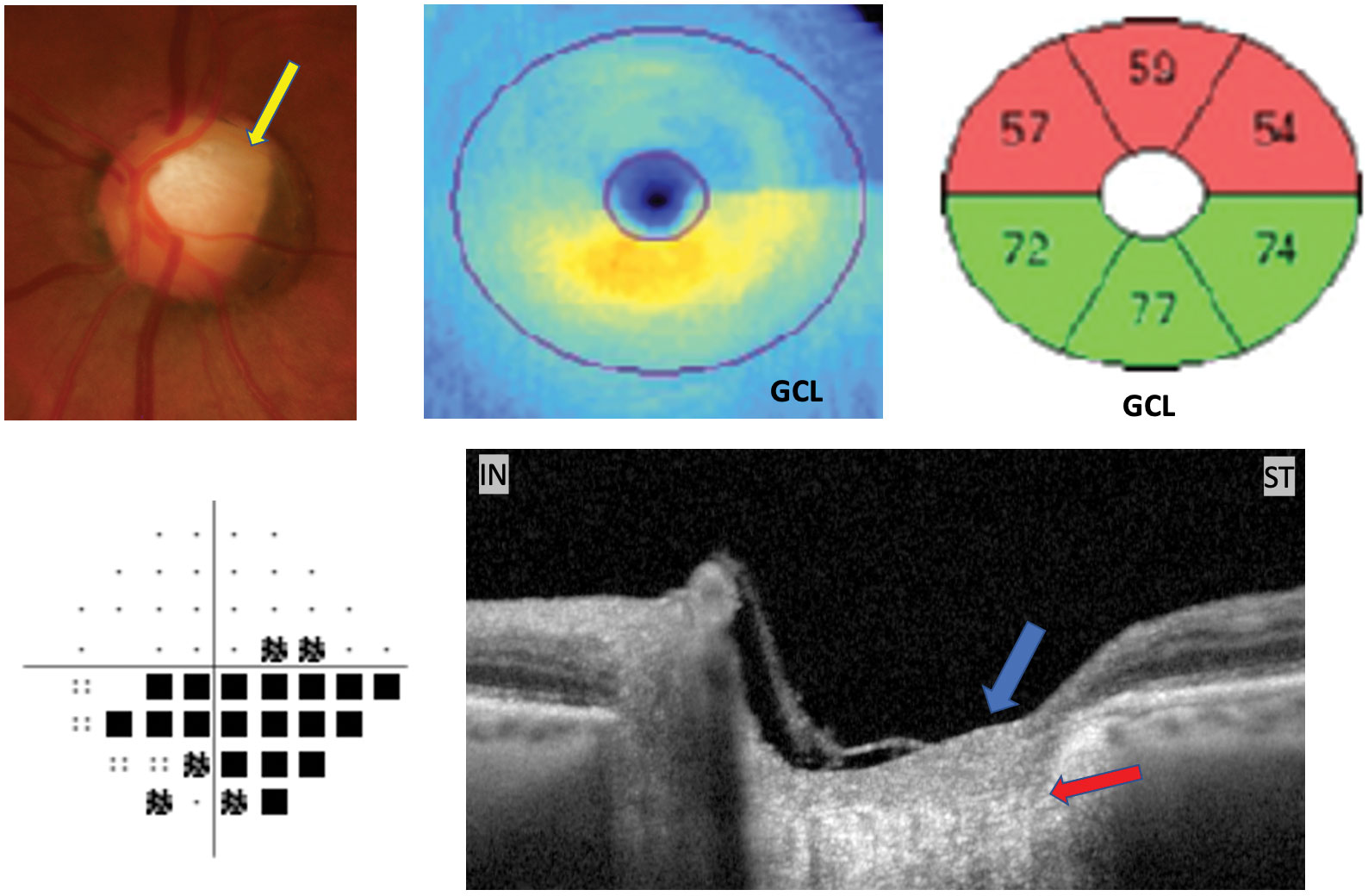 |
| This patient was previously treated for moderate primary OAG OU. They exhibit superior temporal sectoral pallor (yellow pointer), hemispheric ganglion cell loss, inferior visual field defect and intact prelaminar (blue arrow) and laminar tissue (reddish arrow) shown on Oct through the superior temporal rim. This is consistent with AION, non glaucoma. Click epitome to overstate. |
ONHD is considered a subtype of pseudopapilledema and presents with acellular deposits consisting of calcium, amino and nucleic acids and mucopolysaccharides that course in the prelaminar ONH.32 Caucasians and patients with a family history of ONHD have a higher prevalence, and the majority of ONHD cases are bilateral.thirty,33 Researchers reported the all-time supported understanding of ONHD formation by using histological studies to make up one's mind the presence of aberrant axonal metabolism, leading to axonal disruption, extrusion of mitochondria into the prelaminar extracellular space and dysregulation of calcium degradation.32,33
Another study adamant the angioarchitecture in ONHD differs from the normal optic nervus. The authors report that embryologic evolution of optic nervus vasculature is disturbed early by the presence of ONHD, creating risk of vascular flow impedance at the level of the central retinal avenue and its arterial bifurcations within the optic disc. Thus, while ONHD is considered a benign status, there may actually be gamble of vascular obstruction at the optic nerve, inducing chronic hypoxia and long-term RNFL damage.33,34
ONHD and glaucoma may present meantime. Therefore, the get-go step in clinical evaluation is a thorough examination of the optic nerve, its vasculature and the surrounding RNFL. The more superficial the ONHD, the more "lumpy-bumpy" the disc margin, with a nodular, yellowish-to-gray papillary colour. Exist enlightened that the peripapillary retinal vessels are never obscured in beneficial ONHD—an of import distinction from papilledema. The indistinct or elevated disc margins caused by ONHD can mask the appearance of glaucomatous cupping, making funduscopic examination challenging. Equally a result, assessment for wedge defects with the red-costless filter may exist the best funduscopic clue for concurrent glaucoma.30,33
Confirmation of ONHD is a necessity, as misdiagnosis may burden the patient with plush over-management and needless treatment. Recognizing the presence of buried and/or superficial ONHD with appropriate technological work-up will not only rule out emergent diagnoses like papilledema but will also assist in the identification of non-glaucomatous vs. glaucomatous patterns. Fundus autofluorescence (FAF) of the optic nerve will bear witness hyper-autofluorescent, superficial, calcified drusenoid bodies. FAF sensitivity to detect buried ONHD is reported to be every bit low as 12% to 18%.33
B-browse ultrasonography, the traditional gold standard for ONHD identification, will reveal hyper-echoic drusenoid bodies within the optic nerve, but it also has a lower detection rate for buried drusen, likely because they are less calcified.35 So, the clinician may demand enhanced-depth imaging Oct (EDI-Oct) or swept-source Oct (SS-October) of the optic nerve to visualize buried drusen.33,36 EDI-OCT and SS-Oct have been suggested as the new gold standard for visualizing superficial and buried ONHD, which announced as hyporeflective bodies with surrounding hyper-reflective borders.36
With confirmation of ONHD, the next stride is to rule out neuropathic or glaucomatous damage. RNFL thinning is typically localized to the area with greatest ONHD aggregation. The nasal quadrant is the most likely affected section, and RNFL thinning stabilizes as the superficial movement of ONHD concludes. Progressive RNFL thinning over time, especially in the inferior and superior quadrants, may signal glaucomatous rim thinning.
Monitoring the ganglion jail cell layer (GCL) is the near sensitive measure to examine for glaucomatous neuro-retinal damage, as benign ONHD will not cause ganglion cell deficits.33 Visual field results may exist normal (48%), simply defects can occur, especially in superficial ONHD, with varying patterns including enlarged blind spot (30%) and incomplete arcuate or concentric narrowing defect (22%).33,37 The wearisome, progressive nature of visual field defects in ONHD is debated, with etiologies of mechanical pinch or vascular compromise currently under investigation.33,36
While ONHD pathophysiology differs from that of glaucoma, the ONHD construction-function human relationship may involve ONH hypoperfusion and symptomatic visual deficiencies. Currently, in that location is no standard handling for ONHD. Observation over time is preferred with the understanding that elevated IOP with progressive RNFL and GCL loss or visual field defects may betoken concurrent glaucoma and thus warrant the initiation of IOP regulation.33
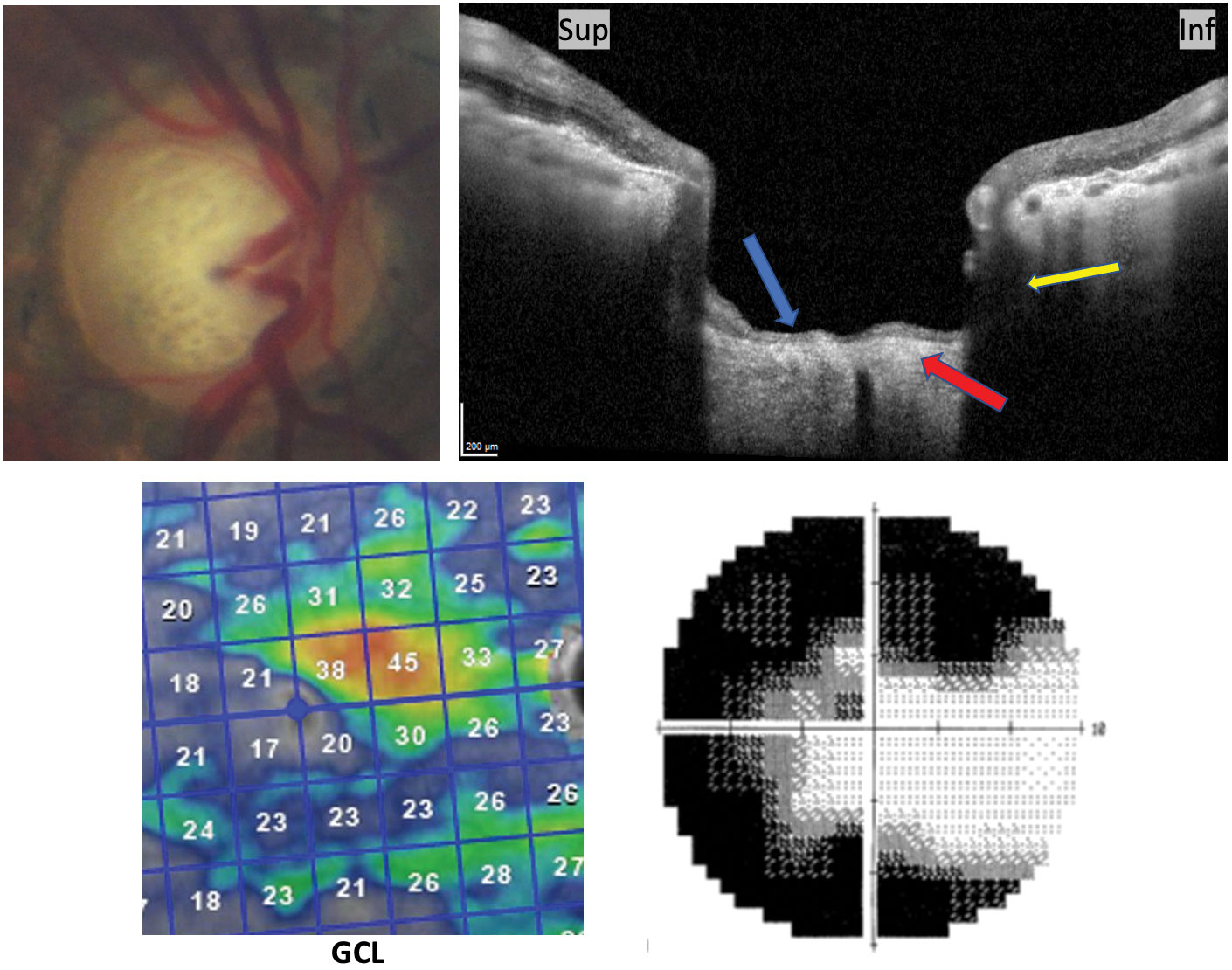 |
| This patient has advanced glaucoma. Notation the posterior and sub-scleral excavation (yellowish arrow) of prelaminar tissues (blue pointer) and lamina thinning (red arrow) on vertical OCT cross-section consequent with glaucoma. Advanced GCL loss and respective 10-2 grayscale are included. Click image to enlarge. |
Papilledema . True papilledema is bilateral optic disc edema due to increased CSFP and subsequent increased intracranial pressure level (ICP). Information technology presents initially with indistinct, hyperemic and elevated rim tissue, potentially with vessel obscuration, hemorrhages, negative spontaneous venous pulsation and Paton'southward lines. Papilledema has diverse systemic etiologies, including compressive brain tumors, venous sinus thrombosis and idiopathic intracranial hypertension (IIH). Confirmation of papilledema from funduscopic and technological examination requires emergent intervention.38
Elevated CSFP in the optic nerve sheath will directly influence laminar position via increased translaminar pressure difference, which produces a cyberspace posterior force on the surface of the lamina and a hydrostatic pressure level gradient within the prelaminar and laminar tissues. If unmanaged, this mechanical insult volition lead to optic nerve swelling from axoplasmic menstruum stasis, peripapillary expansion and deformation and globe flattening. When ICP is decreased with advisable treatment (e.g., weight loss, oral acetazolamide, lumbar puncture, shunt surgery), the CSFP within the optic nerve sheath will likewise decline. Timely management of these cases is critical, as chronic edema tin can lead to irreversible harm.39
Differentiating papilledema from glaucoma comes into play clinically when managing the long-term effects of papilledema, which include structural optic nerve and ganglion prison cell atrophy and functional visual decline. Funduscopically, the optic nerve may appear diffusely pale with no excavation or cupping of the rim tissue. If RNFL atrophy is severe (approaching 40µm in thickness), the optic nerve may have overlying damaged astrocytes, which appear like to glial tissue and may obscure the rim margin.9,31 Post-edema RNFL values vary depending on the chronicity and severity of the papilledema, with some reports of a return to normal ranges and others of mild to drastic atrophy.
October can be very helpful in these cases, with certain parameters offer more than information than others. One study determined elevated ICP is associated with increased ONH volume and decreased Bruch's membrane displacement volume. It found that with a decrease in ICP (after lumbar puncture) comes a decrease in ONH book and in the ganglion cell complex volume, reinforcing how retinal atrophy is too a consequence of optic disc edema. Also of note was the stability of loving cup book before and after lumbar puncture, which supports the funduscopic presentation of optic nervus atrophy without cupping.38
Other inquiry examined IIH patients who were treated with acetazolamide or surgical intervention and discovered that ganglion cell loss occurred on average well-nigh two months after initial diagnosis, despite treatment, which correlated to the extent of final visual field loss and mean deviation reduction even up to one-year mail service-treatment. Thus, from a clinical standpoint, although visual field assessment has been considered the gold standard for monitoring functional loss in papilledema, doctors should now consider series GCA to aid in detection of earlier patterns of neuropathic disease.39
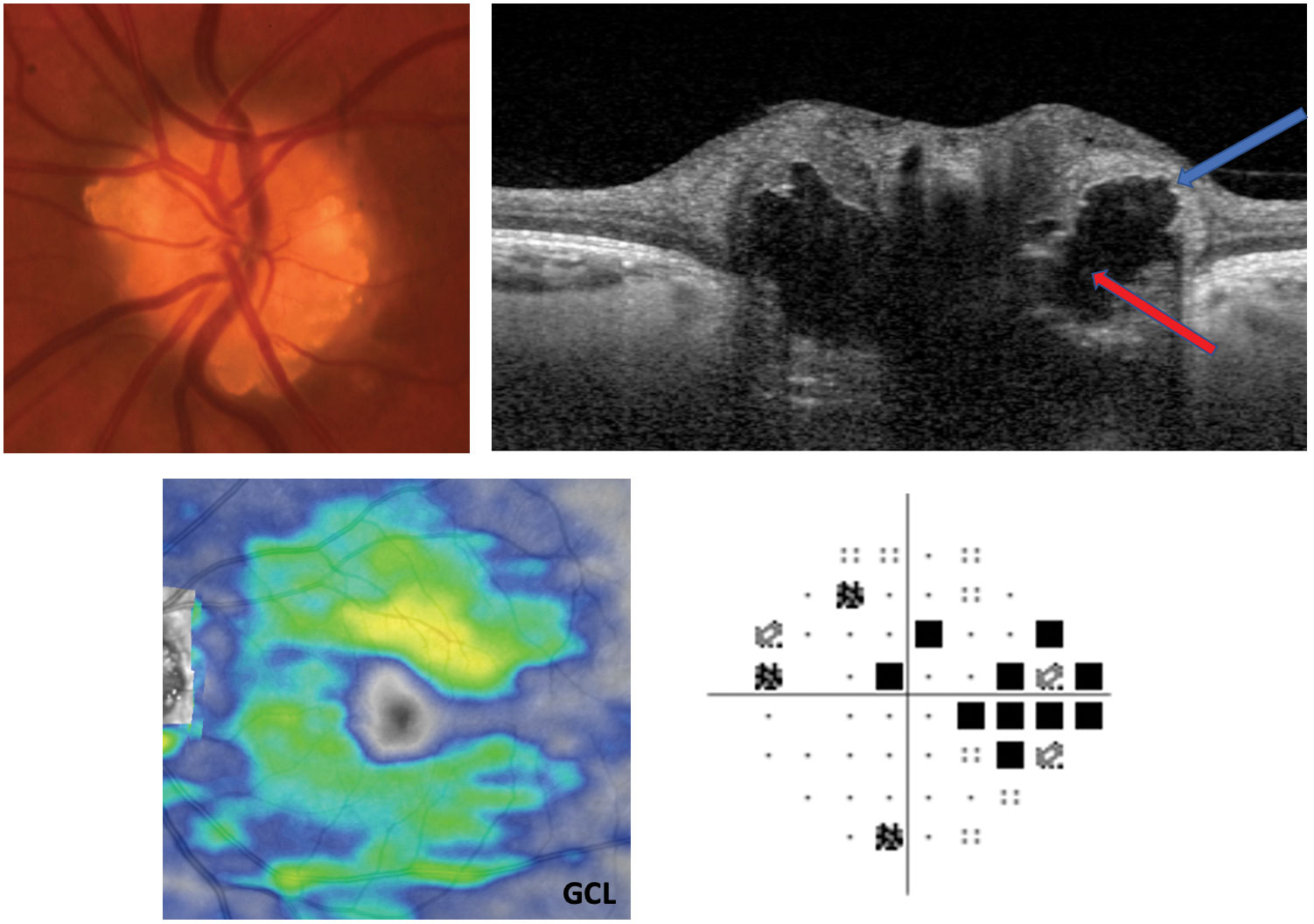 |
| Pictured here is ONH drusen. Note the hyper-reflective borders (blueish arrow) and hyporeflective core of the drusen (red arrow) on EDI-October cross-section. A myriad of presentations tin occur with both visual fields and GCL. This patient's GCL is stable, and Humphrey visual fields show a partial arcuate defect, which is a mutual field outcome. Click image to enlarge. |
Ischemic optic neuropathies . Anterior ischemic optic neuropathies (AION) initially present with optic disc edema from swelling of the RNFL and are classified as not-arteritic (NAION) or arteritic (AAION) based on presentation and etiology. NAION is the nigh frequent cause of unilateral optic disc edema in ages 45 to 70 and is characterized past a hyperemic, edematous nerve with flame hemorrhages and arteriolar narrowing that causes sudden, painless vision loss.twoscore
AAION is an ocular emergency which arises from giant cell arteritis, a systemic vasculitis of medium- and large-sized vessels, with variable systemic symptoms such as jaw claudication and temporal headache. AAION presents with a chalky-white, edematous optic disc, with hemorrhages and severe, sudden, painless vision loss. If astute AAION is suspected, emergent intervention is necessary to reduce the gamble of progression to bilateral blindness.41
Ischemic optic nerve damage is not governed by IOP-related tissue stress similar glaucoma, but instead from a hypoxic state from insufficient posterior ciliary artery apportionment, which cascades retinal ganglion jail cell axonal damage to axonal death. Systemic or nocturnal hypotension and systemic vascular diseases such as diabetes and hypertension may initiate NAION optic nerve non-perfusion.9,xl,42
NAION RNFL damage is greatest in superior disc sectors, specially the nasal and temporal sectors. This is likely explained by more tightly packed axons and thus higher oxygen demand, leading to a more rapid hypoxic state in these sectors.43 AAION pathophysiology involves inflammatory occlusion of the brusk posterior ciliary arteries at the ONH in response to granulomatous vasculitis.41
Regardless of etiology, ischemic insult to the optic nerve will induce pallor and RNFL atrophy without rim tissue excavation or laminar deformation.nine This is clinically observed every bit "pallor greater than cupping," distinguishing the backwash of ischemic insult from glaucoma.42-44 Researchers examined stop-stage AION for the presence of cupping and determined ii% of NAION optics experienced cupping vs. sectoral pallor (72%) or diffuse pallor (28%), while 92% of AAION eyes experienced cupping.43
Optic nerve earthworks may be defective in NAION because the lamina cribrosa is spared from ischemic insult.42 Further topographical investigation with scanning light amplification by stimulated emission of radiation ophthalmoscopy shows a difference in ischemic vs. glaucomatous cupping; neither NAION nor AAION experience deep cupping like glaucoma. Research constitute glaucomatous cup volume was seven times greater than NAION and ii times greater than AAION, confirming that while cup size in cease-stage AAION and glaucoma may appear similar, it is the glaucomatous nerve experiencing progressive posterior excavation and laminar deformation.44
RNFL edema can vary in severity but is more significant and diffuse in AAION than NAION. Every bit the ischemic insult enters its chronic stage (>half-dozen weeks), RNFL edema begins resolving and RNFL atrophy occurs. One study determined that a majority of acute NAION patients experienced superior-quadrant RNFL thickening, with RNFL thinning in chronic NAION predominantly occurring in the superior and temporal quadrants, with pregnant atrophy progressing for up to half dozen months before plateauing. Compared with glaucoma, this RNFL cloudburst is rapid and matches the clinical presentation of divers disc pallor, even approaching OCT "flooring" micron values.
Astute and chronic NAION will present with feature GCA altitudinal loss, virtually ordinarily superior and identifiable earlier optic disc edema resolution.45 Segmenting out the macular layers shows ganglion cell loss occurring every bit early as two weeks post-initial presentation, with progressive inner retinal thinning occurring until it levels out at effectually three months.45 When comparison astute with chronic NAION, the internal plexiform layer (IPL) thinning was the most pregnant of the macular layers.xl,45
This is in direct contrast to glaucoma where less abrupt GCL thinning occurs, IPL thinning is less severe and macular RNFL thinning mimics optic disc RNFL loss.46 Ischemic inner retinal loss corresponds functionally with altitudinal visual field loss, unremarkably junior, some other hallmark finding of acute and chronic NAION.40,47 Generalized GCA and visual field depression is typically experienced in AAION; exist mindful that a patient with severe vision loss >twenty/200 from either ischemic condition may non have the visual capacity to perform an authentic visual field test.40
Takeaways
A host of not-glaucomatous optic nervus conditions either confound examination or create a clinical advent of glaucomatous mimicry. Taking a systematic clinical and technological arroyo, while addressing each patient'due south care on an private footing, will aid differentiate optic nerve conditions and reduce patient burden.
Dr. Hogan is a clinical instructor at Southern College of Optometry. Dr. Rixon is an attention optometrist at the Memphis VA, an AAO diplomate in glaucoma and a fellow member of the Optometric Glaucoma Lodge. Neither author has any financial interests to disclose.
morgansurionotely.blogspot.com
Source: https://www.revieweducationgroup.com/ce/understanding-onh-dynamics-in-glaucoma-and-beyond